Tissue Origin:
Apatos Mix: cortico-cancellous heterologous bone mix
Apatos Cortical: heterologous cortical bone
Tissue Collagen:
Degraded
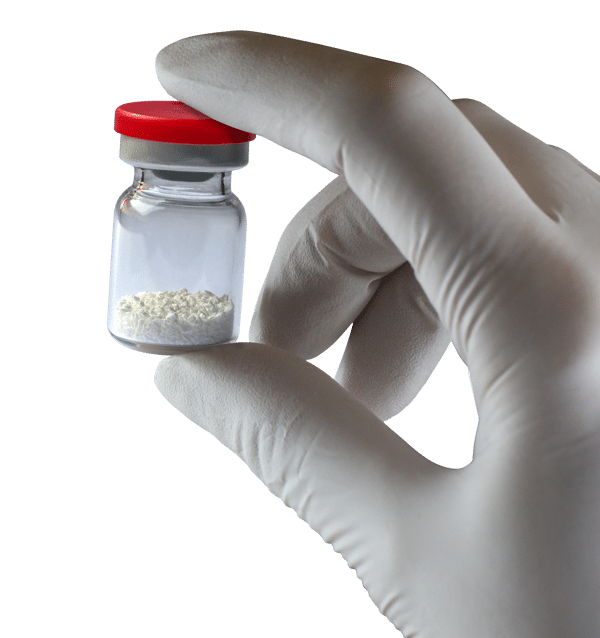
Physical form:
Radiopaque granules of mineral hydroxyapatite
Composition:
Apatos Mix: 100% cortico-cancellous mix
Apatos Cortical: 100% cortical bone
Granulometry:
600-1000 µm
1000-2000 µm
Re-entry Time:
About 5 months
Characteristics:
Apatos is a biocompatible(1,2), osteoconductive(3,4) biomaterial of heterologous origin with characteristics similar to mineralized human bone(5); it can therefore be used as an alternative to autologous bone. The natural microporous consistency of Apatos facilitates the formation of new bone tissue in bone defect area(6), accelerating the process. Apatos microcrystalline hydroxyapatite is available in cortical and mixed granules.
Handling:
Apatos must always be hydrated and thoroughly mixed with a few drops of sterile saline or with TSV Gel to increase graft stability in not self-contained defects; it can also be mixed with patient’s blood.
Finally it can be mixed if necessary with the drug selected for surgery; the mixture thus obtained should be positioned with a sterile spatula or syringe for biomaterials.
Clinical Information:
Apatos is a universal filler, that can be used to treat peri-implant defects and two-wall defects(7,8). Because of its granulometry, Apatos cannot be used in narrow defects, but it fits well in big sockets, e.g. after molar extractions(9). Both sinus lift procedures (with crestal or lateral access)(2,10) can be performed with Apatosas bone substitute, as well as surgeries for horizontal regenerations.
Apatos Cortical is characterized by a very long resorption time(11), guaranteeing adequate preservation of the grafted volume. When needed, Apatos grafts can be protected with OsteoBiol® Evolution membrane(12) or stabilized with Cortical Lamina.
The above clinical information is based on the experience of expert surgeons.
Bibliography:
1 TRUBIANI O, SCARANO A, ORSINI G, DI IORIO D, D’ARCANGELO C, PICCIRILLI M, SIGISMONDO M, CAPUTI S
THE PERFORMANCE OF HUMAN PERIODONTAL LIGAMENT MESENCHYMAL STEM CELLS ON XENOGENIC BIOMATERIALS
INT J IMMUNOPATHOL PHARMACOL, 2007 JAN-MAR; 20(1 SUPPL 1):87-91
2 ORSINI G, SCARANO A, PIATTELLI M, PICCIRILLI M, CAPUTI S, PIATTELLI A
HISTOLOGIC AND ULTRASTRUCTURAL ANALYSIS OF REGENERATED BONE IN MAXILLARY SINUS AUGMENTATION USING A PORCINE BONE-DERIVED BIOMATERIAL
J PERIODONTOL, 2006 DEC;77(12):1984-90
3 BRUNELLI G, SOLLAZZO V, CARINCI F, PALMIERI A, GIRARDI A, MONGUZZI R
OSTEOBIOL® INFLUENCES OSTEOGENIC DIFFERENTIATION OF ADIPOSE DERIVED STEM CELLS
EUR J INFLAMM, 2011, VOL. 9, NO. 3(S), 103-107
4 CAKIR M, KARACA IR, AYSEGÜL F, KAYMAZ F, BOZKAYA S
EXPERIMENTAL EVALUATION OF THE EFFECTS OF ANKAFERD BLOOD STOPPER AND COLLAGENATED HETEROLOGOUS BONE GRAFT ON BONE HEALING IN SINUS FLOOR AUGMENTATION
CLIN ORAL IMPLANTS RES, 2015 MAR-APR;30(2):279-85
5 KOLMAS J, SZWAJA M, KOLODZIEJSKI W
SOLID-STATE NMR AND IR CHARACTERIZATION OF COMMERCIAL XENOGENEIC BIOMATERIALS USED AS BONE SUBSTITUTES
J PHARM BIOMED ANAL, 2012 MAR 5;61:136-41
6 BARONE A, TOTI P, QUARANTA A, ALFONSI F, CUCCHI A, NEGRI B, DI FELICE R, MARCHIONNI S, CALVO GUIRADO JL, COVANI U, NANNMARK U
CLINICAL AND HISTOLOGICAL CHANGES AFTER RIDGE PRESERVATION WITH TWO XENOGRAFTS: PRELIMINARY RESULTS FROM A MULTICENTER RANDOMIZED CONTROLLED CLINICAL TRIAL
J CLIN PERIODONTOL, 2017 FEB;44(2):204-214
7 BARONE A, AMERI S, COVANI U
IMMEDIATE POSTEXTRACTION IMPLANTS: TREATMENT OF RESIDUAL PERI-IMPLANT DEFECTS. A RETROSPECTIVE ANALYSIS
EUR J IMPLANT PROSTHODONTICS, 2006,2: 99-106
8 BARONE A, TOTI P, QUARANTA A, DERCHI G, COVANI U
THE CLINICAL OUTCOMES OF IMMEDIATE VERSUS DELAYED RESTORATION PROCEDURES ON IMMEDIATE IMPLANTS: A COMPARATIVE COHORT STUDY FOR SINGLE-TOOTH REPLACEMENT
CLIN IMPLANT DENT RELAT RES, 2015 DEC;17(6):1114-26
9 BARONE A, TOTI P, QUARANTA A, ALFONSI F, CUCCHI A, CALVO GUIRADO JL, NEGRI B, DI FELICE R, COVANI U
VOLUMETRIC ANALYSIS OF REMODELLING PATTERN AFTER RIDGE PRESERVATION COMPARING USE OF TWO TYPES OF XENOGRAFTS. A MULTICENTRE RANDOMIZED CLINICAL TRIAL
CLIN ORAL IMPLANTS RES, 2016 NOV;27(11):E105-E115
10 IEZZI G, DEGIDI M, PIATTELLI A, MANGANO C, SCARANO A, SHIBLI JA, PERROTTI V
COMPARATIVE HISTOLOGICAL RESULTS OF DIFFERENT BIOMATERIALS USED IN SINUS AUGMENTATION PROCEDURES: A HUMAN STUDY AT 6 MONTHS
CLIN ORAL IMPLANTS RES, 2012 DEC;23(12):1369-76
11 SCARANO A, PIATTELLI A, PERROTTI V, MANZON L, IEZZI G
MAXILLARY SINUS AUGMENTATION IN HUMANS USING CORTICAL PORCINE BONE: A HISTOLOGICAL AND HISTOMORPHOMETRICAL EVALUATION AFTER 4 AND 6 MONTHS
CLIN IMPLANT DENT RELAT RES, 2011 MAR; 13(1):13-18
12 MARCONCINI S, GIAMMARINARO E, DERCHI G, ALFONSI F, COVANI U, BARONE A
CLINICAL OUTCOMES OF IMPLANTS PLACED IN RIDGE-PRESERVED VERSUS NONPRESERVED SITES: A 4-YEAR RANDOMIZED CLINICAL TRIAL
CLIN IMPL DENT RELAT RES, 2018 DEC;20(6):906-914








There are no reviews yet.